Clinical Evidence
Pivot-TR has demonstrated strong first-in-human results, achieving significant TR reduction, right heart remodeling, and symptom improvement in high-risk patients with severe secondary TR. Building on these outcomes, Tau Medical is expanding clinical trials and compassionate-use programs for Pivot Extend® and Pivot Bridge® across multiple sites in South Korea, Australia, Canada, Japan, India, and Germany.
Study Overview
Study Type
Multi-center, single-arm, short-term implantation
Centers
11 hospitals across South Korea
Sample Size
15 patients (out of 19 screened)
Patient Profile
Severe symptomatic secondary TR
Implant Duration
≤ 7 days (temporary implantation)
Analysis
Core lab-reviewed echocardiography and cardiac CT
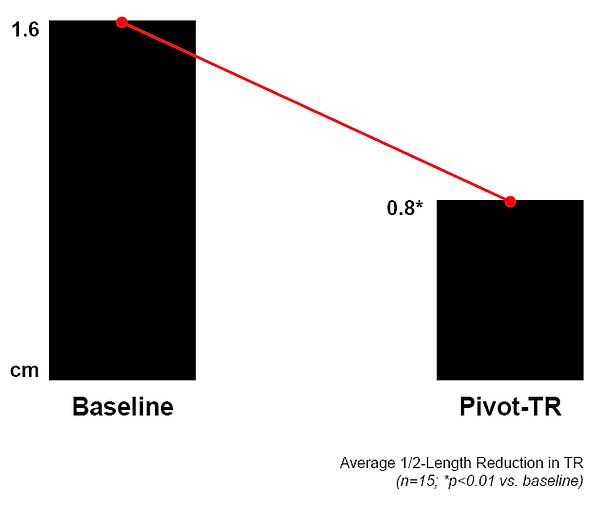
50% Average Length Reduction in TR
Vena Contracta — Pivot-TR
50% Average Area Reduction in TR
EROA*— Pivot-TR
*Effective Regurgitant Orifice Area

Post Procedure TR Grade Reduction
1 Grade Reduction
2 Grade Reduction
3 Grade Reduction
Anatomical Stabilization

8.1% Avg. RV Volume Reduction

10.2% Avg. TV Annulus Reduction
Clinical Stabilization

•NYHA Class Improvement (NYHA 2.5 -> 1.5, p<0.05)
•Body Weight : 1.5 Kg ↓
•eGFR : 10%↑ (p<0.05)
Learn More
For more information about the Pivot-TR platform and Tau Medical’s ongoing programs, please email us directly at: INFO@tau-medical.com.
Compassionate Use
If you are a patient, caregiver, or clinician seeking more information about access to
Pivot Bridge® or Pivot Extend® through a Compassionate Use or Expanded Access program, we welcome your inquiry. Tau Medical is committed to supporting patients with severe tricuspid regurgitation who may not qualify for conventional treatments.
Please use the form below to tell us more about your situation, and a member of our clinical team will respond shortly.
